CoVarr-Net Biobank, Data Platform & Bioethics
About Us
In 2025, the CoVaRR-Net Biobank, initially established at the Ottawa Hospital Research Institute (OHRI) in 2021, evolved into a pathogen-agnostic resource under the new name Canadian Biobank Alliance (CBA). The CBA plays a key role in enhancing pandemic preparedness for several infectious diseases and non-infectious health threats. Together with its Data Platform and Bioethics Units, the CBA continues to facilitate the rapid and secure sharing of biological materials, data and microbes with academic researchers, government agencies and industry partners nationally and globally.

A Unique National Biobank System
The CBA is a pioneer as Canada’s only national biobank system, connecting biobanks across the country to facilitate resource sharing and collaboration.
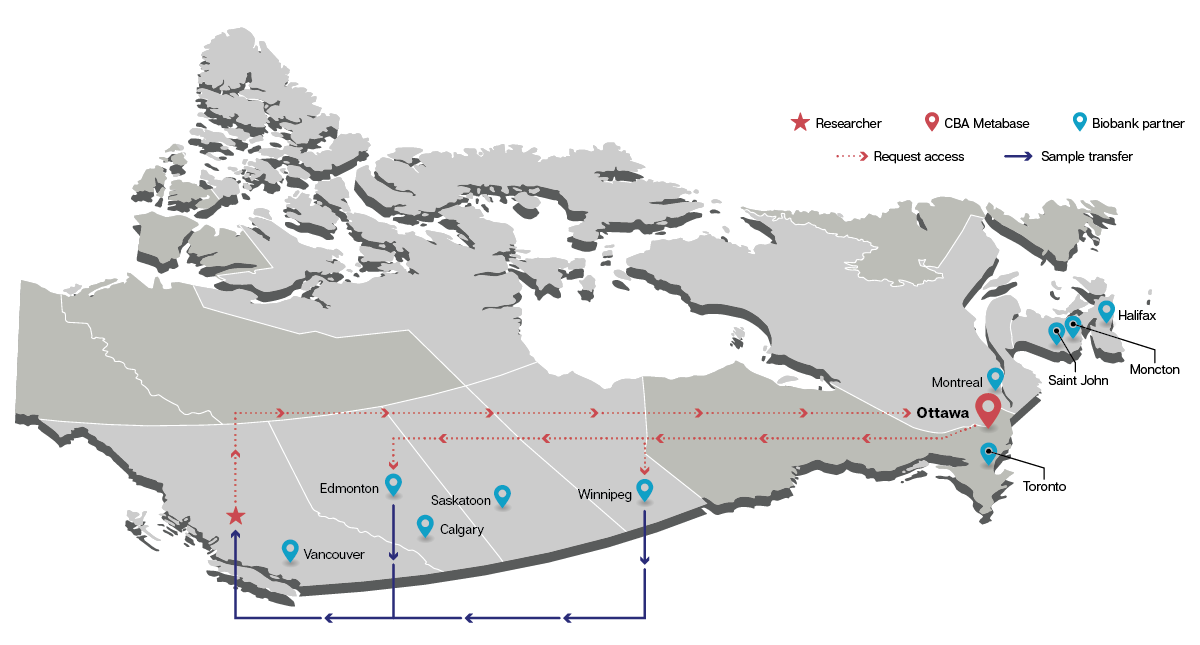
The CBA’s lead site, based in Ottawa, facilitates resource applications and sharing through a federated model that allows individual biobanks to retain autonomy over their materials and data. During the COVID-19 pandemic alone, the CBA network collected over 500,000 specimens from more than 50,000 Canadians. As the CBA moves beyond SARS-CoV-2, the scope of accessible resources will continue to expand.
Diverse Collection of Samples and Pathogens
In addition to SARS-CoV-2-related samples, the CBA has expanded its collection to include research on various pathogens such as mpox, avian influenza (H5N1), group A streptococci (GAS), and the respiratory syncytial virus (RSV). Known as a pathogen-agnostic biobank, it is prepared to adapt to new and emerging pathogens. The CBA can collect and inventory a wide variety of specimen types:
Whole blood
Plasma
Blood cells
Serum
Nasal swabs
Viruses
Breast milk
Stool
Saliva
Tissue
Urine
Wastewater
Cerebrospinal fluid
Environmental pathogens
Peripheral blood mononuclear cells
The CBA strives for inclusive representation and prioritizes Equity, Diversity, Inclusion and Indigeneity (EDI&I) in the collection of specimens and data. In accordance with the First Nations Information Governance Centre’s Principles of Ownership, Control, Access and Possession (OCAP®), the CBA is committed to working and learning together with Indigenous communities and researchers, such as CoVaRR-Net’s Indigenous Engagement, Development and Research Pillar 7 (CIEDAR).
Known as a pathogen-agnostic biobank, it is prepared to adapt to new and emerging pathogens.
Searching for Samples across Canada: the Metabase
The CoVaRR-Net Data Platform has developed a customized national data catalogue, called Metabase, which integrates inventory data from all CBA partner sites. This system enhances efficiency by streamlining and accelerating access to samples and data, all while maintaining robust governance and the highest standards.
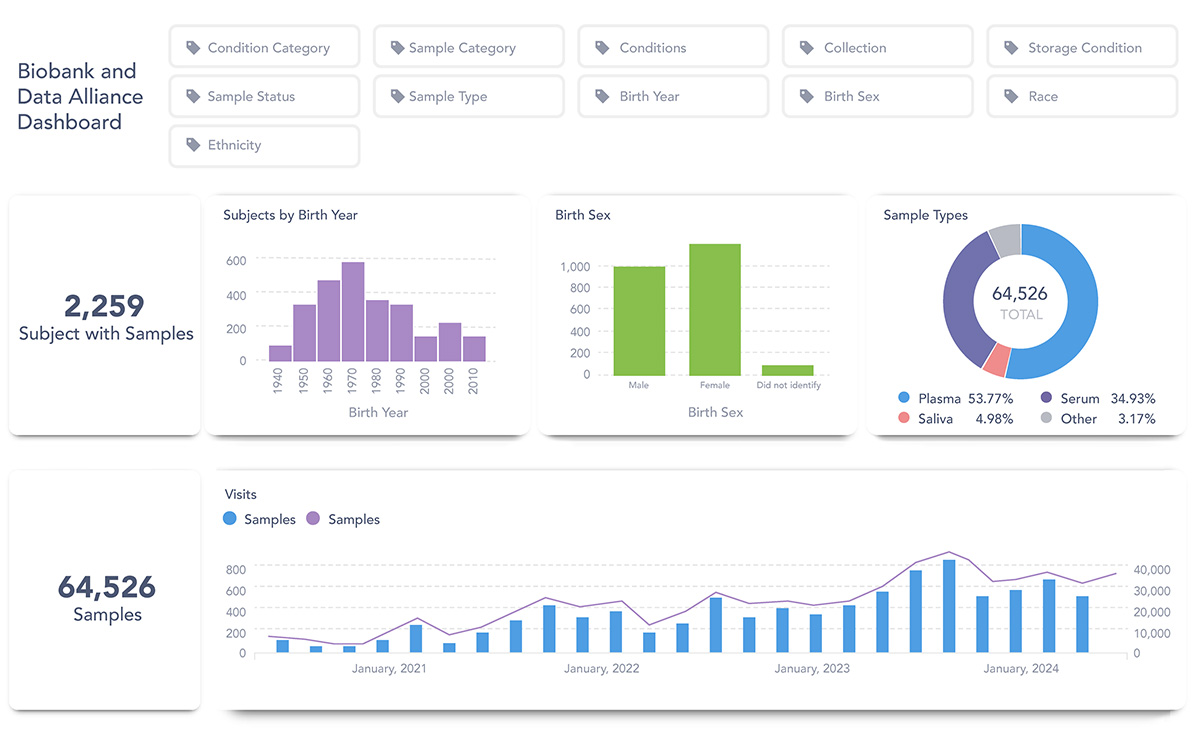
An example of search results within the Metabase
The Metabase’s dashboard allows interested researchers to search the sample repository catalogue and database using a base set of query criteria. Researchers interested in accessing the resources found in the Metabase must complete a four-step process to request access to samples.
Ethical and Contractual Considerations in Biobanking
To enable the seamless sharing of biosamples, data, reagents, and microbes among Canadian academic institutions, the Universal Data and Biological Transfer Agreement (UDBMTA) was developed by the CoVaRR-Net Biobank in partnership with the University of Ottawa and the OHRI. This agreement, vetted by over 30 Canadian institutions, has served as the foundational template for the governing Data and Sample Sharing Agreement (gDSSA) — a new materials transfer document that has broader use and can be applied nationally. Having a ready-to-use MTA process is critical to the preparedness mission of the CBA, helping avoid any delays to the sharing of scientific data or materials.
Additionally, the CoVaRR-Net Bioethics Unit has streamlined multi-jurisdictional research ethics approval procedures by adopting the Clinical Trials Ontario (CTO) template, providing a tool for rapid, minimal-risk research and facilitating inter-institutional collaborations across Ontario. It is an important, pre-established tool for use by the CBA to serve as a lead biobanking entity for multi-jurisdictional academic research, clinical trials and beyond — and critical for preparedness.
Stronger Together: Preparedness Biobanking
The CoVaRR-Net Biobank, now the CBA, has been a leader in advancing preparedness biobanking efforts in Canada for the past four years. Ever grateful for the support of the Canadian Institutes of Health Research (CIHR), the CBA continues to strive for sustainability as Canada, and the public/private sector, move towards a model of national pandemic preparedness.
Going forward, the CBA will focus its efforts on collaborating with partner sites, academic institutions, government agencies, and industry partners. These efforts will further enhance the capacity to share resources, data, and ethical practices in biobanking across the country, ensuring continued collaboration in support of preparedness research and public health.
Contact the CBA
Our Team
CoVaRR-Net Biobank
Nikita Rayne
Biobank Manager
Aliisa Heiskanen
Coordinator







